UDSv4 Updates
One of the largest and most comprehensive longitudinal, standardized, clinical and neuropathological datasets in the world!
About NACC's Uniform Data Set

Introducing Version 4 of the Uniform Data Set (UDSv4)
The UDS was introduced in 2005 to standardize longitudinal neurocognitive data collection across NIA’s Alzheimer's Disease Research Centers (ADRC) Program currently comprised of 37 centers. NACC convenes a Clinical Task Force, comprised of leading dementia experts from across the US, every few years to develop a new version of the UDS that is aligned with important scientific advances in Alzheimer's Disease and Related Dementia (ADRD) research, detection, and care.
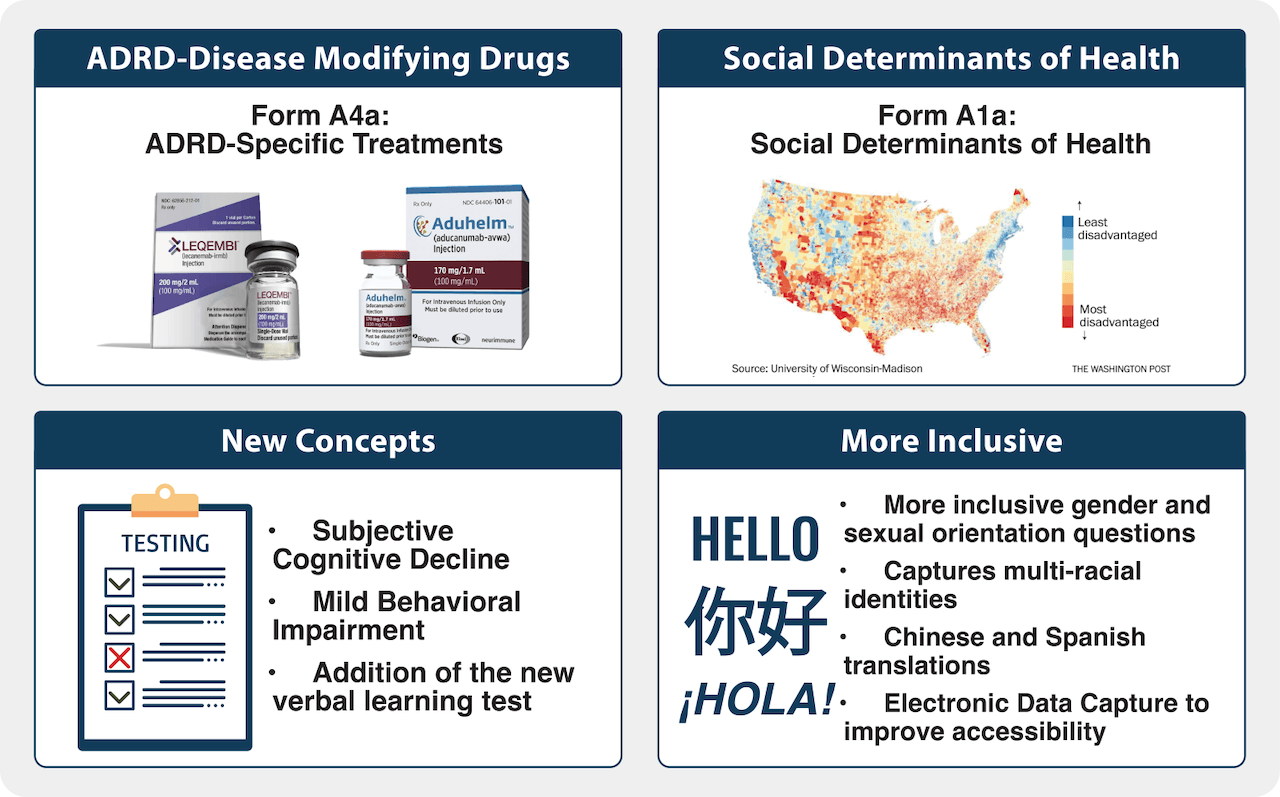
The last update to the UDS (v3) was released in 2015. Since then, the AD/ADRD field has made significant progress making it critical to update the UDS once again. UDSv4 will introduce a form focused on social determinants of health (SDOH) as well as updated, more inclusive questions on gender identity, sexual orientation, and multi-racial and -ethnic identities which will enable researchers to explore new and critical questions related to diversity, equity, and inclusion (DEI) research. NACC and the CTF strive to foster inclusion with these questions as well as promoting best practices for engaging indigenous communities across the ADRCs, providing Spanish and Chinese translations of the UDS, and implementing electronic data capture (EDC) to improve accessibility for UDS participants.
UDSv4 will also advance the AD/ADRD field with a new form focused on AD/ADRD-disease modifying drugs as well as new concepts throughout the UDS including subjective cognitive decline, mild behavioral impairment, and a verbal learning test to assess new cognitive domains.
Collaborations
Co-Creating the new UDS: NACC Partners with the Clinical Task Force (CTF)
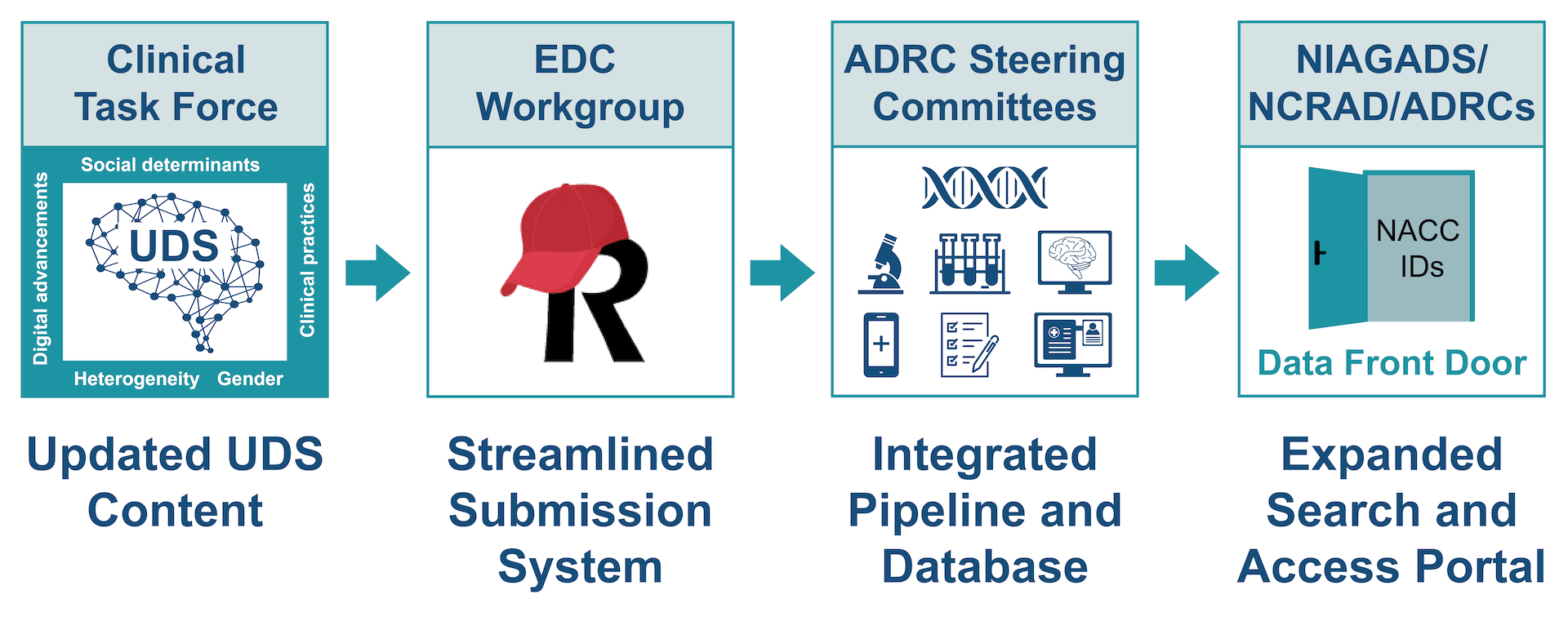
UDS updates are critical for advancing the field, and we are honored to collaborate closely with the Clinical Task Force on this effort. All this work is happening under the leadership of and in close collaboration with the Clinical Task Force led by Dr. Allan Levey. NACC collaborates with the CTF on meeting agendas, setting strategic goals, and tracking deliverables. The CTF also includes a series of workgroups focused on specific specialties.

NACC is collaborating with the ADRC Electronic Data Capture (EDC) Workgroup to build a modern, streamlined, and scalable EDC System for UDSv4 that will effectively serve the needs of the community. The EDC Workgroup was launched by NACC in collaboration with the Data Core Steering Committee in January 2022. The EDC Workgroup is currently comprised of 97 members from across 33 ADRCs who are collaborating across three subgroups to co-develop REDCap forms, define requirements, and to develop documentation and training materials for UDSv4.
Modernization
NACC Modernization in Support of UDSv4
- A modern Electronic Data Capture system powered by REDCap
- Transparent and accessible data quality checks
- A cloud-based multimodal data integration and harmonization platform
- A new advanced data search, visualization, and access interface for all ADRC data accessed via the NACC Data Front Door
Developing a Modern Electronic Data Capture System Powered by REDCap
NACC is actively updating its existing submission system to create a streamlined electronic data capture platform for the ADRC Program.
Transparent and accessible data quality checks
NACC is creating a streamlined process for submitting and validating UDSv4 form data compared to the current system. NACC will post Excel files listing the data quality rules in a human-readable format to GitHub and will also post these data quality rules in JSON format in the same repository. Additionally, NACC will provide the Python code that will be used to run the data quality checks upon submission via a GitHub repository.
Building a Modern Cloud-based Multimodal Data Integration, Harmonization, and Sharing Platform
NACC is transitioning to a new modern cloud-based multimodal data integration, harmonization, and sharing platform that will not only accommodate UDSv4 forms but also effectively scale to collect and integrate new data streams to provide a robust long-term resource for the research community. All ADRC data streams will be connected to NACCIDs and searchable through the NACC Data Platform.
This new NACC Data Platform is described in more detail here
Support for ADRCs
Supporting ADRCs through the transition to UDSv4
To aid ADRCs with the transition to UDSv4 and the new data submission options accompanying the forms update, the following resources will be provided:
- Onboarding Checklist - step-by-step instructions with links to every resource ADRCs need to get started
- Live Trainings / Webinars - NACC in collaboration with the CTF and EDC Workgroups will offer both live and recorded clinical and data manager training webinars
- Office Hours - unstructured Zoom meeting times where ADRC members can join and ask questions of NACC's Technology, REDCap, and Research Teams
- Moderated ADRC UDSv4 Community Forum (powered by Discourse) - amoderated forum for ADRCs and NACC to support sharing information, collaborating, and getting answers to your questions
- UDSv4 FAQ - a living, changing list of the most common questions and answers will be available on Discourse and through a link on the NACC Website
- Github Library - copies of REDCap forms, error checks, coding guidebooks, and other resources per form
- REDCap SOPs - instructions and best practices, including action tags, calculated fields, cross-project piping, custom reporting, data access groups, data quality rules, data dictionaries, survey development and distribution, customization of user roles, how to use Crosswalks, JSON files, and XML, and more
- UDSv3 to UDSv4 Crosswalks - data harmonization instructions between UDSv3 and UDSv4 for each form
- REDCap Forms - two different resources: 1) the NACC-hosted REDCap environment, or 2) available for download as XML files from the Github Library
UDSv4 Pilot
NIA, NACC, and the CTF have worked over the past several years to update the UDS forms from version 3 to version 4. The changes, which impact most of the UDS forms, are complete and have begun being piloted at ADRCs. This pilot is an excellent opportunity for sites to become familiar with the new UDSv4 forms and practice submitting data before it becomes a requirement in Fall 2024. In addition, pilot sites can provide valuable feedback regarding the Coding Guidebooks, which are the instruction manuals for clinicians and testers administering the updated forms. NACC and the CTF will consider this feedback before the formal UDSv4 roll-out.
UDSv4 Webinars and Presentations
May 14, 2024 UDSv4 Virtual Clinical Training Workshop
March 2024 Operationalizing "Impaired, not MCI" in UDSv4 Webinar
This hour-long webinar addressed operationalizing "Impaired, not MCI" vs MCI in the UDSv4. We opened with an overview of the item on UDSv4 Form D1a. Each speaker gave a case presentation, with ample time at the end for discussion and audience Q&A. This webinar was hosted by NACC and the ADRC Clinical Core Steering Committee as a part of our UDSv4 Webinar Series. Watch here
February 2024 UDSv4 Cognitive Task Force Webinar
Presenter Dr. Kostas Lyketsos uses a case-based approach to ratings of neuropsychiatric symptoms and mild behavioral impairment on Form D1a and related forms. Background to the concept of MBI and its importance as an early symptom of dementia is also shared. Watch here
Updates
Email Updates
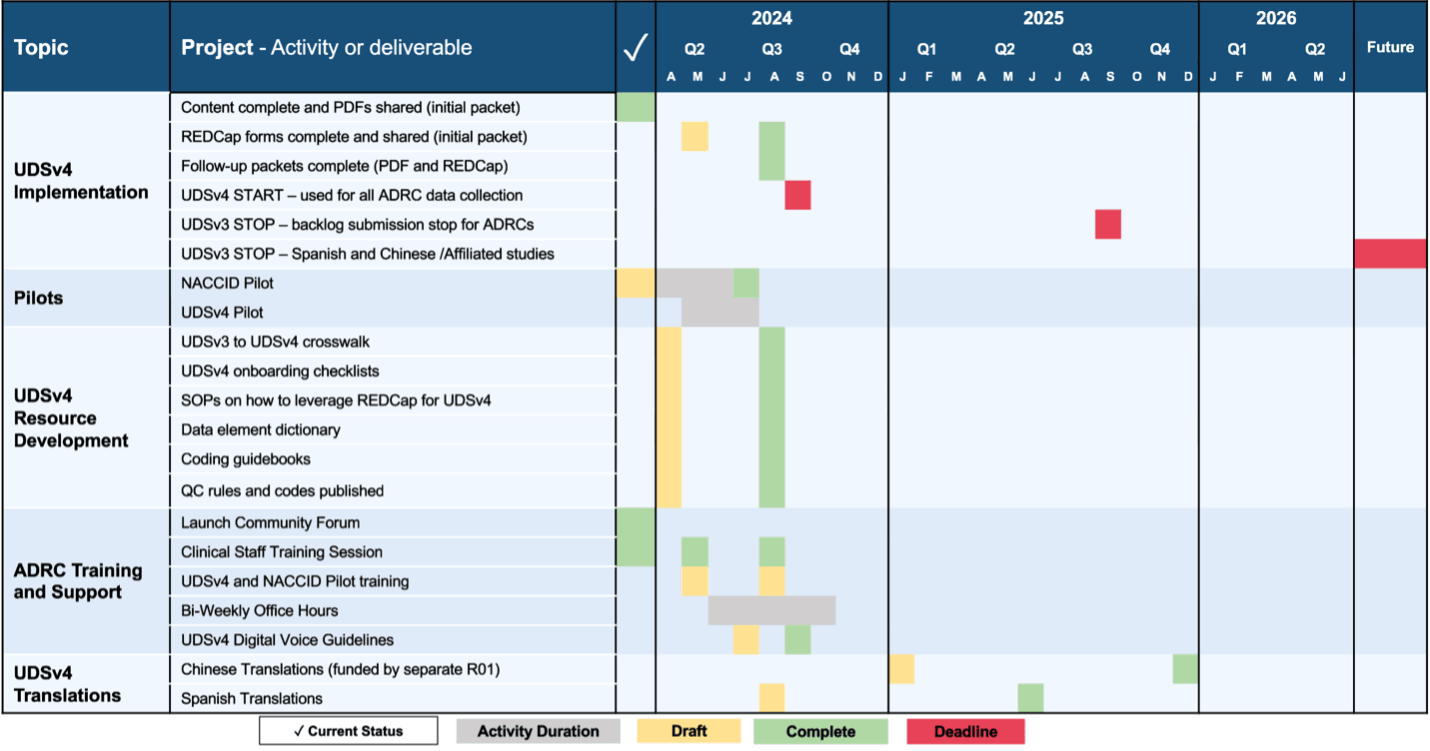
UDSv4 Development Timeline
Preview UDSv4 Forms
As NACC and our partners are finalizing the content and supporting infrastructure for UDSv4, we are making forms and documentation available early to enable those collecting and using UDS data to start preparations for the new form data. NACC and the CTF are launching the UDSv4 Pilot in order to identify any improvements that are needed in the UDSv4 guidance documentation, and the final versions will be available once this pilot concludes to align with the UDSv4 Implementation Timeline. Please see this page for more information about the UDSv4 Pilot.
| Form | Form Name | CTF Status | |
|---|---|---|---|
| A1 | Participant Demographics | Approved | FormA1-Preview-April2024.pdf |
| A1a | Social Determinants of Health | Approved | FormA1a-Preview-April2024.pdf |
| A2 | Co-Participant Demographics | Approved | FormA2-Preview-April2024.pdf |
| A3 | Participant Family History | Approved | FormA3-Preview-April2024.pdf |
| A4 | Participant Medications | Approved | FormA4-Preview-April2024.pdf |
| A4a | AD-Specific Drug Treatment | Approved | FormA4a-Preview-April2024.pdf |
| A5/D2 | Participant Health History | Approved | FormA5D2-Preview-April2024.pdf |
| B1 | Evaluation Form: Physical | Approved | FormB1-Preview-April2024.pdf |
| B3 | UPDRS-Parkinson's | Approved | FormB3-Preview-April2024.pdf |
| B4 | Global Staging-CDR: Clinical Dementia Rating | Approved | FormB4-Preview-April2024.pdf |
| B5 | Behavioral Assessment: NPI-Q | Approved | FormB5-Preview-April2024.pdf |
| B6 | Behavioral Assessment: GDS | Approved | FormB6-Preview-April2024.pdf |
| B7 | Functional Assessment: FAS | Approved | FormB7-Preview-April2024.pdf |
| B8 | Evaluation Form: Neurological Examination Findings | Approved | FormB8-Preview-April2024.pdf |
| B9 | Clinical Judgement of Symptoms | Approved | FormB9-Preview-April2024.pdf |
| C2 | Neuropsychological Battery Test Scores | Approved | FormC2-Preview-April2024.pdf |
| C2 Worksheets | C2 Worksheets | Approved | FormC2Worksheets-Preview-April2024.pdf |
| C2T | Neuropsychological Battery Test Scores | Approved | FormC2T-Preview-April2024.pdf |
| C2T Worksheets | C2T Worksheets | Approved | FormC2TWorksheets-Preview-May2024.pdf |
| D1a | Clinical Diagnosis | Approved | FormD1a-Preview-April2024.pdf |
| D1b | Biomarker and Etiological Diagnosis | Approved | FormD1b-Preview-April2024.pdf |

